The spore loses its characteristic constituents, and heat resistance decreases substantially. In the final phase h2o is taken up, and metabolism (synthesis of ATP, proteins and genetic materials) resumes. Warmth activation is an important Consider the incidence of the shoulder during the survival curve of bacterial spores upon heating.
New approaching webinar: Navigating pharmaceutical environmental checking in the altering marketplace! Sign up your particulars right now!
Observing colony morphology and differing kinds of fungal staining approaches is utilized to determine yeast and molds.
This cookie is about by YouTube. Utilized to trace the information of the embedded YouTube videos on a website.
With state-of-the-artwork services and skilled microbiologists, Creative Diagnostics specializes in offering extensive microbial limit testing solutions customized to your unique demands of varied industries.
Precise and well timed documentation of this process is vital for traceability and regulatory compliance.
The microbial limit test of Organic medicines will involve evaluating the microbial contamination present in the final drug solution. Organic medication, specially These derived from biological resources or made working with biotechnological procedures, are susceptible to microbial contamination during production, packaging, or storage.
Applicability test strains of mould and yeast counting methods: Candida albicans and Aspergillus niger. The managed click here bacteria inspection technique is to check regardless of whether there are particular microorganisms within the test item under specified test situations. These are culture medium suitability inspection and bacterial Regulate inspection strategy suitability test respectively. Test strains to the applicability in the Manage bacteria counting process: bile-resistant Gram-detrimental microorganisms, Escherichia coli, Salmonella, Pseudomonas aeruginosa, Staphylococcus aureus, Clostridium, and Candida albicans.
We make no illustration or warranty regarding the accuracy of the data contained in the joined web sites. We propose that You usually verify the knowledge acquired from connected read more Sites before acting upon this information.
Audits assist establish places for advancement and be sure that testing routines align with the Firm’s quality management procedure.
From a pure microbiological viewpoint putting on an Over-all doesn’t seem sensible other than the advertising of the Perspective of Doing work cleanly and neatly. Currently immediately after 1–two h the overall bears as much contamination as the private outfits. Instructions for garments are however also essential to encourage occupational basic safety and wellbeing (see Sect.
Endotoxin tests don't involve culturing, so a report is produced within a day. Concurrently, bioburden testing demands culturing and can take nearly 7 to 10 days for reporting.
Give comprehensive schooling on incubation parameters, like temperature and period. Highlight the value of maintaining these problems to aid microbial growth and correct colony formation.
Within this action, the overall amount of aerobic organisms is determined, which is an important indicator to evaluate the hygienic quality of medicines.
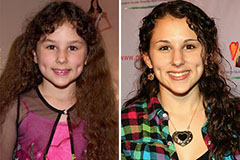 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Anthony Michael Hall Then & Now!
Anthony Michael Hall Then & Now! Mason Reese Then & Now!
Mason Reese Then & Now!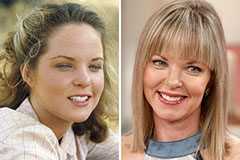 Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now! Rachael Leigh Cook Then & Now!
Rachael Leigh Cook Then & Now!